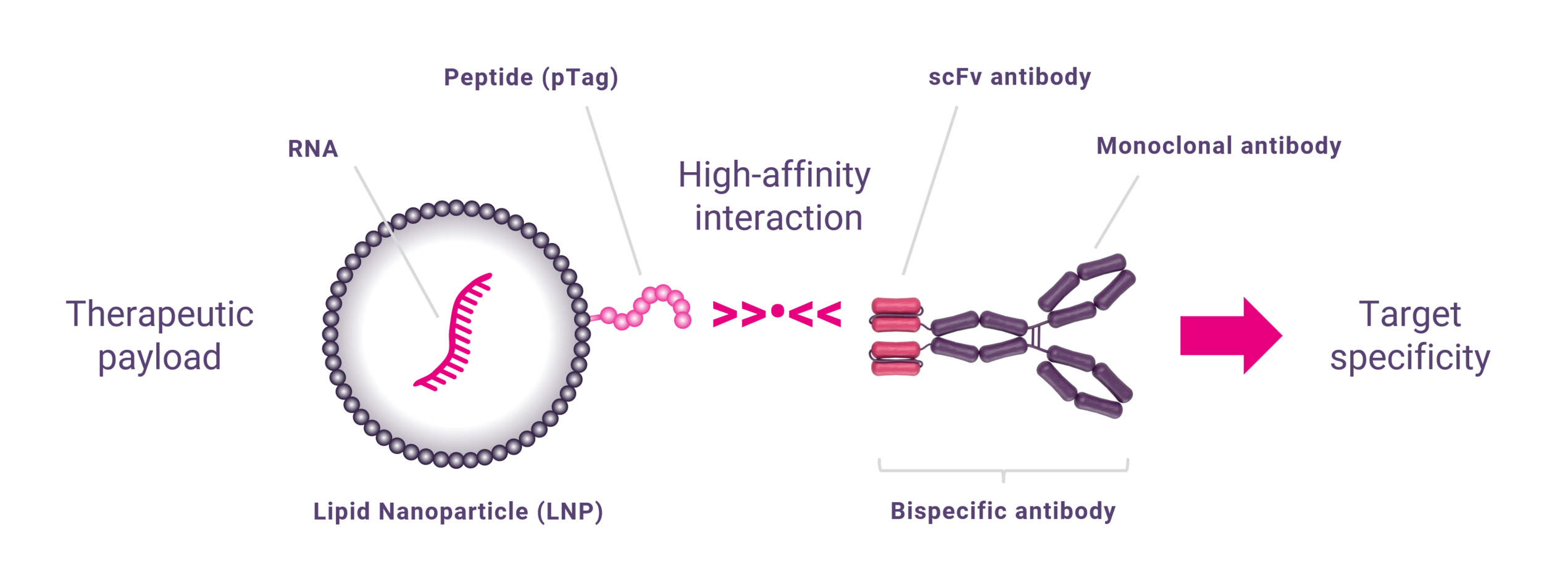
Technology platform for targeted delivery of nanoparticles
The core technology builds on a high-affinity interaction between a proprietary synthetic peptide tag (pTag) and scFv antibody fragment. The interaction is unique in several ways. The pTag is very short, only 9 amino acids long, and lacks secondary structure, making it highly predictable and developable. The affinity is in the low picomolar range, and displays signs of induced fit, meaning that the initial binding is replaced by a very strong binding with almost irreversible properties. The pTag can be fused with many different payloads, such as peptides, antisense oligonucleotides, small molecules, and notably, lipid nanoparticles, LNPs.
By attaching the pTag to the surface of LNPs, and the scFv to a targeting unit, such as an antibody, forming a bispecific antibody, bsAb, and enabling targeted delivery of the LNP. The LNP-pTag-bsAb complex is formed by affinity interaction by mixing and the target specificity is provided by the non-scFv part of the bsAb. In principle, the scFv can be attached to any targeting unit allowing delivery of LNPs to any cell carrying a cell-specific surface antigen. Moreover the LNP can be loaded with any RNA, which in theory allows reprogramming of any cell in the body. The technology platform is especially promising for its potential within the field of in vivo CAR-Ts. To this end Strike is developing a lead in vivo CAR-T candidate that delivers a CD19 CAR to T cells based on a CD3 targeting antibody.